|
Department
chemistry of complex compounds
Novel coordination compounds of platinum metals with O-, N-, S-containing
physiologically active molecules, derivatives of hydrazones, amines,
salicylaldimines, thiourea and iminethiazoles.

In this series of investigations, we aimed our efforts at synthesizing novel
coordination compounds of platinum metals with a number of physiologically active
molecules-ligands and studying their physicochemical properties and structure to
find out whether they can be used in medicine. The functionally derivatives of
hydrazones 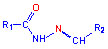 ,
amines ,
amines  ,
azomethines (salicylaldimines) ,
azomethines (salicylaldimines) 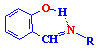 ,
thiourea (for example thiosemicarbazones (TSCNs)) ,
thiourea (for example thiosemicarbazones (TSCNs)) 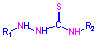 and iminethiazoles were chosen by us as physiologically active ligands.
and iminethiazoles were chosen by us as physiologically active ligands.
Why gust these objects?
Platinum metals.
The chemistry of platinum metals has always attracted researchers attention,
which is primarily due to the wide range of their oxidation states, which can
be realized for these elements in compounds. A consequence is a huge number
of synthesized and investigated coordination compounds of different structure
and hence with different properties.
As is well known the platinum metals compounds and there materials have valuable
physicochemical properties requisite for the wide use of them in various fields
of science and technology: catalysts, protective coatings, biologically active
compounds for medicine, etc is of no small importance.
The purposeful synthesis of ruthenium, rhodium and palladium complexes is conduct
in our Institute. And then we investigate them in logical order:
SYNTHESIS→COMPOSITION→STRUCTURE→PROPERTY→FUNCTION.
With this going on the composition and structure of compounds are determine
by complex modern analysis: elemental analysis, infra-red spectroscopy (IR),
UV-Vis spectroscopy, X-ray photoelectron spectroscopy (XPS), diffuse reflection
spectra (DRS), heteronuclear NMR spectroscopy
(NMR 1H, 13C, 14N, 195Pd,
COSY 1H-1H, 1H-13C), thermogravimetry
and X-ray structural analysis. The compoundТs function investigates in two
directions:
- As the analytical forms for the division and extraction of ruthenium, rhodium and palladium from natural and industrial raw materials.
- As the precursors of new drugs for the cardiovascular and/or cancer therapy.
In recent decades, a new trend has appeared in the chemistry of platinum metals Ц
biocoordination chemistry, which is at the junction between such branches of science
as coordination chemistry, biochemistry, biophysics, medicine. Now, the antitumoral
activity of platinum compounds is well known, however a search for more active and
less toxic preparations based on other platinum metals (for example ruthenium,
rhodium and palladium) with multifaceted bioactivity is constantly under way.
The high efficiency of some ruthenium complexes in the photodynamic therapy of
tumors has been established. Anticancer and antimetastatic activity of the complex
of ruthenium with indazole and phthalocyanides are known, for which a high activity
in the case of autochtonous colorectal cancer diseases of rats has also been
established. It is supposed that Ru(III) complexes act as prodrugs, chansing into
corrssponding active form as a result of reduction in vivo. On the basis of
palladium compounds, a drug (efasol) has been developed, which has an
immunomodulatory action which facilitates immune response in the immunodeficiency
state of the organism, e.g. after irradiation. Some palladium compounds possess
antiphage activity, inhibit membrane-bound, and show histamine releasing and
histamine binding properties.
Ligand systems.
The derivatives of hydrazones, amines, azomethines (salicylaldimines or Schiff
bases), thiourea (thiosemicarbazones(TSCNs)) and iminethiazoles belong to
polydentate ligands since they contain quite a number of electron donor atoms of
the functional groups (OH), (C=O), (C=S), (NH), whose ability for donor-acceptor
interaction varies as a function of synthesis conditions and the properties of
the central complexing atom. The fact of existence of these molecules in the form
of various tautomers is of no small importance. The possibility to fix various
tautomeric ligand forms during complex formatio, the number of possible conformation
and configuration tautomers and isomers increasing when modifying donor atoms and
complicating the carbonyl or azine fragment of hydrazones, is of scientific
interest. At the same time they are polybasic acids. This range of chemical
peculiarities makes it possible to synthesize with their participation manifold
coordination compounds with different structure and properties. These compounds
are undoubtedly of interest for practical purposes as well.
The use of them in agriculture and medicine is mentioned, where they have promise
in the treatment of quite a number of diseases: extrapulmonary tuberculosis,
tonsillitis, influenza, smallpox, poliomyelitis. Antifungal and fungicidal
activity of azomethines and their metallocomplexes has been established, which
increases when there is a hydroxyl group in the ligand molecule. The complexes
with tetra-and tridentate Schiff bases exhibit, along with anti-inflammatory and
bactericidal properties, antineoplastic action towards the ascitic type of
EhrlichТs carcinoma, Walker 256 carcinoma, 180 sarcoma.
Thiosemicarbazones (TSCNs) are very promising molecules in biocoordination
chemistry because of their pharmacological properties which include notably
their antiparasital, antibacterial and antitumor activities. Some
thiosemicarbazones increase their antitumor activity by their ability to
form chelates with specific metallic ions. These ligands are formed by the
condensation of a thiosemicarbazide and an aldehyde, and both of them can
create another coordination site. The thiosemicarbazone ligand usually
coordinates with a metal through the imine nitrogen and the sulfur atom.
The ligands feature more than two covalent sites, the number of which depends
on the aldehyde, and on the tautomeric equilibrium of the thiosemicarbazone,
although the most common way to coordinate is through the thiolic form. Evidence
of this feature can be found in the tremendous volume of transition metal chemistry
that has been published for these ligands, in some cases involving tridentate
coordination.
Iminethiazoles are inhibitor of factor induced by extremal hypoxia. At the same time
they have antitumor action due to ability for slow down tumor cells growth.
To understand the mechanism of action and transport in the organism of biolosically
active coordination compounds, it is necessary to know, first of all, the processes
of complex formation of platinum metals with ligands containing pharmacophore atomic
groups. The above-mentioned azomethines, hydrazones and carbothioamides belong to
such ligands.
The above properties of some complexes aroused our interest in the development of
synthesis methods and in the investigation of the physicochemical and biochemical
properties of new coordination compounds of nobel metals, which can contribute to
the development of understanding of the interrelation between the structure and
bioactivity of compounds for possible, in prospect, goal-directed synthesis of new
compounds with predictable properties.
What have we done in this direction?
1. Synthesis methods have been developed; tens of new coordination
compounds of ruthenium, rhodium, palladium and platinum with derivatives of
hydrazones, amines, azomethines and carbothioamides have been synthesized and
isolatet in individual state. It has been determined there composition, structure
and physicochemical properties. The general regularities involved in the complex
formation of platinum metals with N-, O-, S-containing ligands and factors that
affect the composition, structure and physicochemical properties of the
coordination compounds obtained have been determined.
It has been found:
-
for example for Ru(III), Rh(III), Pd(II) with hydrazone derivatives,
that depending on synthesis conditions (medium pH, concentration and stoichiometry
of original substances, synthesis time and temperature) and the nature of
substitnents in ligands, four-, five- or six-coordinate compounds of ruthenium,
rhodium and palladium of molecular or ionic (anionic, cationic) type with ligand
coordination both in monodeprotonated amide cis- or trans-form and in bideprotonated
imidol form are formed. The realization of the amide or imidol form of hydrazone
in the complex depends on synthesis medium pH. The formation of mono-and binuclear
complex compounds depends on other synthesis conditions. Ru(III) and Rh(III)
complexes have a pseudooctahedral structure with ligand coordination in bi-
or tridentate cyclic fashion through oxygen/sulfur atoms of hydroxyl (OH) group
and carbonyl/thio-((C=O)/(C=S)) or imidol/thiol ((C-O)/(C-S)) group and through
the azomethine atom of nitrogen. The effect of electron donating and electron
withdrawing substituents in hydrazone molecules on the complex formation of
ruthenium and rhodium has been studied. It has been found that complexes of
ionic type are partly or complectely soluble in water, whereas hydrazones
themselver are insoluble.
-
In the case of preparing compounds with salicylaldimine and carbothioamide
derivatives, the synthesis conditions, which influence greatly the formation
of complexes of different composition and structure and the form of coordinated
ligand, also play a key role. By varying synthesis conditions, complexes both in
the form of chelates and in the form of adducts can be obtained. In this case,
either adducts or chelates of ionic type only dissolve in water.
In these complexes, the ligand is coordinated as a chelating anion in the bidentate
chelating mode via the oxygen atom of the deprotonated OH group and the azomethine
nitrogen atom. The presence of the intramolecular hydrogen bond in the ligand
molecule and the palladium affinity for nitrogen favored the formation of the
palladium(II) complex in which the neutral ligand is monodentately coordinated
via the pyridine nitrogen atom. However, more severe synthesis conditions led
to the palladium complex with the bidentate chelating ligand similar to the
rhodium and ruthenium complexes. As distinct from the latter, the palladium complex
has a square-planar coordination core and is molecular, which is likely responsible
for its poor solubility in alcohols and its insolubility in water. Allocation of
the carbothioamide group in α-position to the pyridine ring of the some
carbothioamide derivatives enables to coordinate it through the pyridine nitrogen
and atom sulfur of thioamide group by forming a six member metallocycles. Variation
of the synthesis conditions (pH, temperature, heating time) takes effect on a number
and form of the coordinated ligand molecules. The thiourea derivatives are coordinate
in thione tautomeric form in all newly synthesized complexes in acid medium.
Therefore, generally cationic complexes are formed. They are soluble in water,
which enable to investigate their biological activity.
It has been proved that the effect of the nature of ligand on the formation
of coordination compound is due to the different spatial arrangement of the
nucleophilis donor centers of the fuctional groups of ligand and to the presence
of an intramolecular hydrogen bond in it. Increase in the number of donor centers
in the ligand molecule not only affects the denticity, of ligand, but also makes
its chanse into another form possible; this was exemplified by the interaction of
Pd(II) with a derivative of carbothioamide, which underwent cylization under
synthesis conditions with the formation of triazole ring.
2. It has been shown that anionic complexes can be effectively used for for
the division and extraction of ruthenium, rhodium and palladium from natural and
industrial raw materials. in the presence of some nonferrous metals. It turned
out that different complex formation rates and different optimum pH ranges of
the reaction medium are characteristic of ruthenium, rhodium and palladium; this
was utilized by us to develop a highly sensitive and selective procedure for the
extraction spectrophotometric determination, division and extraction of ruthenium,
rhodium and palladium from the case of their joint presence in mixtures of their
solutions salts and electrolytes.
Besides, it has been conducted a series of biochemical investigation:
That is for what purpose?
It has been mentioned higher, the study of rhodium and ruthenium complex formation
reactions during transformation of organic compounds gives a key to the
understanding the mechanisms of many important biological processes. The presence
in organometallic compounds of different functional groups and pyrimidine
heterocycles, which play a key role in metabolic processes in living organisms,
provides for high biological activity of both ligands themselves and complexes.
The presence of such conjugated multiple bonds in compounds facilitates
unpaired-electron delocalization. Therefore, the complex compounds can be
efficient scavengers for different kinds of ROS, which are formed in organisms
in various stress situations.
The U.S. investigator D. Harman has emphasized the significance of the ROS in
damage. In particular, he associated the onset of radiation disease, cancer,
atherosclerosis and some liver diseases with ROS-induced injuries. F.Z. Meyerson
has found that membrane lipid peroxidation contributes greatly to the development
of stress-induced injuries. The antioxidants administration prevents many of such
injuries. Accumulation of stress factors affects the cardio-vascular system:
decreases a myocardial contractility, appearance of excitation and conductance
disturbances and development of arrhythmias. In aging the reliability of
antioxidant defense system decreases and the role of the ROS-induced damage
increases. This generates a need for a search the new substances that would
possess a membrane-stabilizing, calcium- mobilizing action and be able to defend
the cell structures from damaging factors.
Therefore, the aim of our bioinvestigation is to develop, on the basis of
fundamental research, methods for the synthesis of novel platinum metals
coordination complexes with oxygen-, nitrogen-, and chalcogen-containing,
aromatic, pyrimidine, thiazole, and heterocyclic organic ligands having an
antioxidant, angioprotective, cardiotonic, and calcium exchange activity for
the correction of cardiovascular disturbances under extreme conditions. The
novel platinum metals coordination complexes may be used for regulation of Na,
K, and Ca balance and correction of the rheological properties of blood.
It has been established that some compounds of ruthenium and rhodium
with salicylaldimines and hydrazones possess, depending on it their structure
and the type of coordinated ligands, demonstrate the antioxidant properties
like vitamin E or trolox, i.e. when they act on damaged isolated myocardial and
vascular preparations. They restore their constriction responses to electrical
stimulation. Besides, changing the stoichiometric metal-ligand ratio leads to a
change in both chemical and biological properties of complexes. According to
preliminary data, the complexes have a cardioprotective action on the
ischemia/reperfusion model in isolated heart. In experiments on an isolated
myocardial trabecula and vascular preparation, these compounds are able to
reduce disturbances of the contractile function, induced by phenylarsine oxide
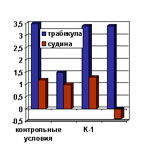
(mitochondrial pore opening stimulator), and restore the contractile activity
of damaged myocardial trabecula (figure 1).
The complex compounds can restore endothelium-dependent regulatory reactions of
preparations, which is very important in the correction of age-specific shifts in
vascular tonicity. It is possible that a stimulator of these processes is the
complex itself and not its decomposition products, and that increasing the
stability constant of complexes will enhance their biological activity. The
introduction of the water-soluble complex obtained by us prevents, most
likely suppiesses partially, the opening of mitochondrial pores and reduces
thereby the effect of activator on them, enabling the heart to come into
normal operation. The reason of this is probably the ability of metal to
form coordination compounds with organic ligands, owing to which the polarity
decreases, and their passive transport across cell membranes is facilitated.
Thus, the compounds investigated by us can be potentially efficient components
in the creation of new cardiotonic drugs.
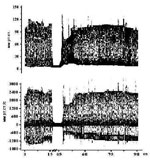
Indices change of cardiodynamics at ischemia/reperfusion of laboratory rats
isolated heart in controlled conditions.
(Table open in new window.)
 |
 |
| Figure 1. Retractive reactions of isolated trabecule and vascular in response of synchronous current stimulation (K-1, 2-rhodium complex with hydrazone derivatives). |
Figure 2. Changes of pressure in the left ventricle of heart over the ischemia/reperfusion model at the allotment of ruthenium complex with salicylaldimine. |
Key researchers:
Candidates of Chemical Sciences Orysyk Svitlana I., Bon Volodymyr V., Rybatchyk Larysa M.
|
|





 ,
thiourea (for example thiosemicarbazones (TSCNs))
,
thiourea (for example thiosemicarbazones (TSCNs))